One label, one SMS, one verification! – this is our authentication process, further, it is what we promise our partners and the public consumers on brand medicines and vaccines consumed.
We recently launched our blockchain platform; http://safe.uthabiti.org/ and through signed partnerships, we will work with health products manufacturers, funders and/or policymakers to label medicines across their supply chains. This has not only increased accountability across their supply chains but also, the safety of health products as they move from stage to stage in the product’s market.
Technology is progressive and Africa in general needs systems that support the use of emerging technologies to solve our local problems. To understand blockchain, it is a growing list of records, called blocks, which are linked using cryptography. Each block contains a cryptographic hash of the previous block, a timestamp, and transaction data. This is how we assure our partners and communities that their health products are safe.
At the point of consumption, communities use our FREE SMS platform number; +12018491388 to SMS the products Unique Asset Identifiers which are revealed before consumption through scratch to reveal sections on each label.
We have run our pilots; with one County Government and one SRH health products supplier. We have targeted communities living within crowded urban spaces and are most vulnerable to counterfeits. Through their mobile phones, they can now check specific brands on their authenticity. Just 3 months of operation and we have run up to 3500 verifications, we are confident that we can scale this across the pharmaceutical players in Kenya.
We are looking for partners and players who are interested in safe proofing the movement of medicines in Kenya. We are interested in serializing all medicines in different supply chains in Kenya, this empowers us to hold pharmaceutical stakeholders accountable for the safety of health products in each supply chain.
If we centralized authentic medicines data/labels, then we can progressively save communities from falling victim to unsafe health products. Our science believes in an end to end management of medicines data, and empowering communities to check for their authenticity, straight from their phones. This has built conversations around the introduction of track and trace regulations in the pharmaceutical products market, this, legally binds providers and manufacturers to publicly avail their data to consumers upon request.
On 4th April 2019, the at Kenya Healthcare Federation (KHF) ICT and Data exchange subcommittee had an engagement with Prof. Francis Ndemo of University of Nairobi (UoN) and Pharmacy and Poisons Board (PPB), to discuss and understand the development of standard codes for medicines. In his opening remarks, Prof. Ndemo informed the meeting that the Ministry of Health is interested in monitoring usage of drugs in the country through a track and trace solution from point of entry to patient.
This has continuously shown the need for Uthabiti’s solution across Kenya.
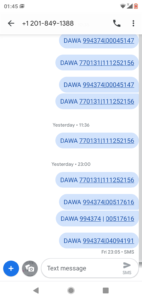
Scratch to reveal codes are verified through the SMS platform
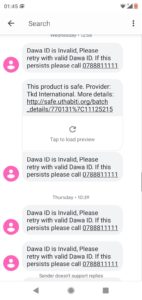
Instant replies are made to the patient with information on the product’s safety
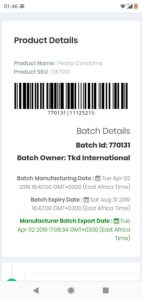
More information on medicine can be accessed and traced back to the manufacturer.
For more information visit:
https://uthabiti.org/
Email: [email protected]
Call us today; +254788811111
Follow us,
Twitter; @Uthabiti
Facebook; Uthabiti
This project is part of the IACC Social Entrepreneurs, an initiative hosted by Transparency International.


